WITHAFERIN A CAS#: 5119-48-2; ChemWhat Code: 44895
Identification
| Product Name | WITHAFERIN A |
| IUPAC Name | (1S,2R,6S,7R,9R,11S,12S,15R,16S)-6-hydroxy-15-[(1S)-1-[(2R)-5-(hydroxymethyl)-4-methyl-6-oxo-2,3-dihydropyran-2-yl]ethyl]-2,16-dimethyl-8-oxapentacyclo[9.7.0.02,7.07,9.012,16]octadec-4-en-3-one |
| Molecular Structure | 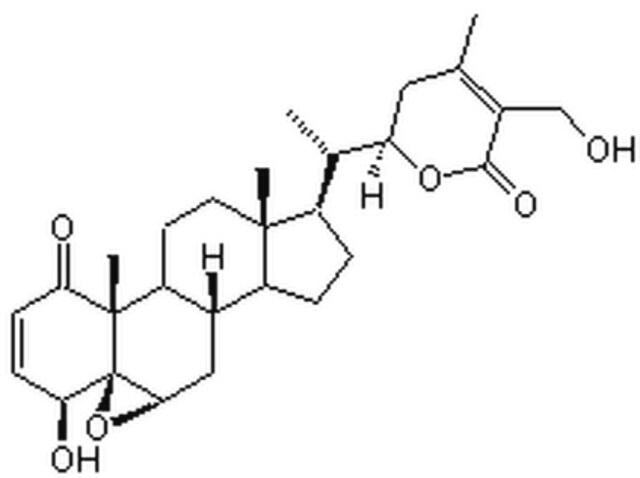 |
| CAS Registry Number | 5119-48-2 |
| EINECS Number | 207-322-2 |
| MDL Number | MFCD00006400 |
| Beilstein Registry Number | 105692 |
| Synonyms | 3-Pyridinamin;3-Pyridinamine;3-Pyridinamine;pyridin-3-amine;T6NJ CZ;3- Aminopyridine;3-Amino-pyridine;3-pyridylamine;Amino-3 pyridine;m-Aminopyridine;MS/MS-1064463;Pyridin-3-ylamine;Pyridine, 3-amino-;β-Aminopyridine 462-08-8 |
| Molecular Formula | C28H38O6 |
| Molecular Weight | 470.60 |
| InChI | InChI=1S/C28H38O6/c1-14-11-21(33-25(32)17(14)13-29)15(2)18-5-6-19-16-12-24-28(34-24)23(31)8-7-22(30)27(28,4)20(16)9-10-26(18,19)3/h7-8,15-16,18-21,23-24,29,31H,5-6,9-13H2,1-4H3/t15-,16-,18+,19-,20-,21+,23-,24+,26+,27-,28+/m0/s1 |
| InChI Key | DBRXOUCRJQVYJQ-CKNDUULBSA-N |
| Canonical SMILES | CC1=C(C(=O)O[C@H](C1)[C@@H](C)[C@H]2CC[C@@H]3[C@@]2(CC[C@H]4[C@H]3C[C@@H]5[C@]6([C@@]4(C(=O)C=C[C@@H]6O)C)O5)C)CO |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| WO2019/116071 | COMPOUNDS FOR TREATING NEURODEGENERATIVE DISORDERS | 2019 |
| US2019/315798 | WITHANOLIDES USEFUL FOR THE TREATMENT OF NEURODEGENERATIVE DISEASES | 2019 |
Physical Data
| Appearance | White to off white powder |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
| Melting Point, °C | Solvent (Melting Point) | Comment (Melting Point) |
| 253 | ||
| 243 – 245 | CHCl3, ethyl acetate | Decomposition |
| 248 – 249 | methanol |
Spectra
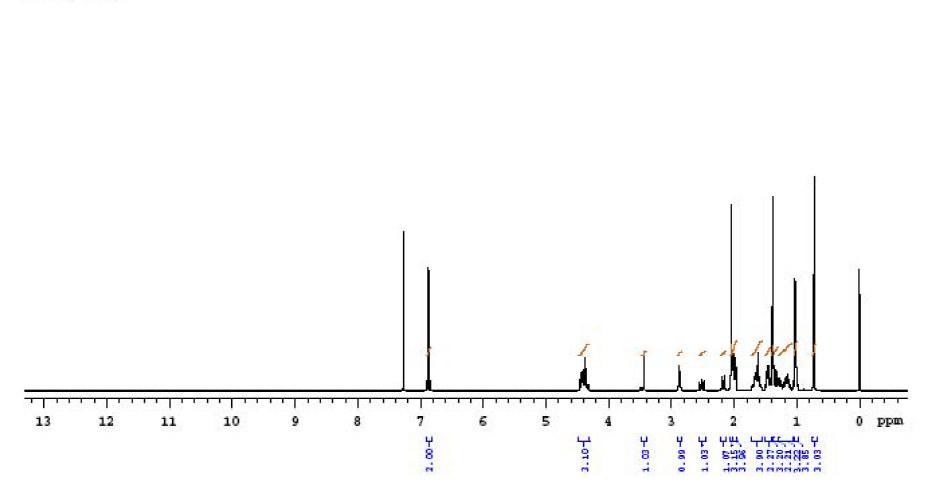
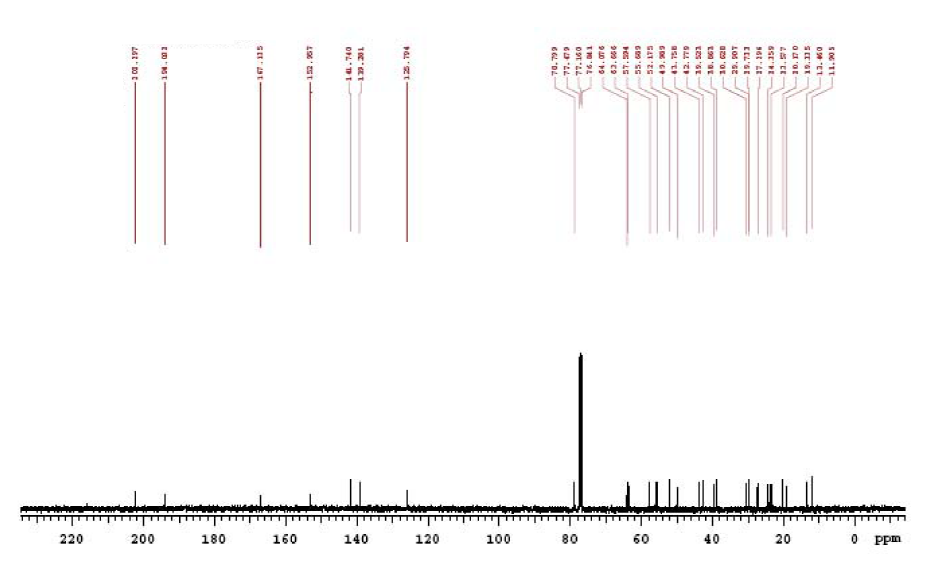
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 100 | |
| Chemical shifts | 1H | chloroform-d1 | 400 | |
| MAS (Magic-Angle Spinning), Chemical shifts, Spectrum | 1H | water-d2 | 19.84 | |
| DEPT (Distorsionless Enhancement by Polarisation Transfer), Chemical shifts, Spectrum | 13C | chloroform-d1 | 125 | |
| Spin-spin coupling constants | CDCl3 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands, Spectrum | potassium bromide | |
| Bands | film | |
| Bands | nujol |
| Description (Mass Spectrometry) |
| liquid chromatography mass spectrometry (LCMS), electrospray ionisation (ESI), time-of-flight mass spectra (TOFMS), tandem mass spectrometry, spectrum |
| electrospray ionisation (ESI), liquid chromatography mass spectrometry (LCMS), spectrum |
| liquid chromatography mass spectrometry (LCMS), tandem mass spectrometry, spectrum |
| HRMS (High resolution mass spectrometry), ESI (Electrospray ionisation), IT (ion trap), CID (collision-induced dissociation), Tandem mass spectrometry, Spectrum |
| ESI (Electrospray ionisation), TOFMS (Time of flight mass spectrum), QIT (quadrupole ion trap), Spectrum |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| Spectrum | ||
| CHCl3 | 213, 238 |
Route of Synthesis (ROS)
| Conditions | Yield |
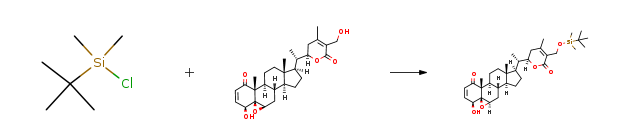
| With 1H-imidazole; dmap In dichloromethane at 20℃; for 3h; | 94% |
| With 4-PP In N,N-dimethyl-formamide at 60℃; for 3h; | 90% |
| With 1H-imidazole; dmap In dichloromethane at 20℃; for 2.5h; | 89% |
| With dmap; triethylamine In dichloromethane at 20℃; for 12h; Reagent/catalyst; |
Safety and Hazards
| GHS Hazard Statements | Not Classified |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | No data available |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 470.606 |
| logP | 3.987 |
| HBA | 6 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 96.36 |
| Rotatable Bond (RotB) | 3 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Pharmaceuticals |
| inducing depletion of tumor-induced bone marrow myeloid-derived suppressor cells of polymorphonuclear type (PMN-MDSCs) |
| treating a cancer in combination with oncolytic virus and adjuvant |
| treating a cancer in combination with oncolytic virus and chimeric antigen receptor (CAR)-expressing T-cells (CAR T-cells) |
| treating melanoma |
| General chemicals |
| quality control, consistency and accuracy of the PV formulation |
| mitigating, alleviating or improving Alzheimer’s disease |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |