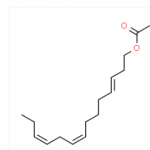
(Z,E)-9,12-TETRADECADIENYLACETATE CAS#: 30507-70-1; ChemWhat Code: 326837
Identification
| Product Name | (Z,E)-9,12-TETRADECADIENYLACETATE |
| IUPAC Name | tetradeca-9,12-dienyl acetate |
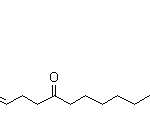
| Molecular Structure | 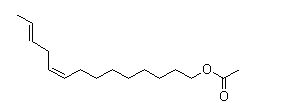 |
| CAS Registry Number | 30507-70-1 |
| Synonyms | Tetradec-9Z,12E-dien-1-yl Acetate;CAS Number: 30507-70-1 |
| Molecular Formula | C16H28O2 |
| Molecular Weight | 252.392 |
| InChI | InChI=1S/C16H28O2/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-18-16(2)17/h3-4,6-7H,5,8-15H2,1-2H3/b4-3-,7-6+ |
| InChI Key | ZZGJZGSVLNSDPG-WWVFNRLHSA-N |
| Canonical SMILES | C/C=C\C/C=C/CCCCCCCCOC(=O)C |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2013/231499 | SYNTHESIS OF Z-OLEFIN-CONTAINING LEPIDOPTERAN INSECT PHEROMONES | 2013 |
| US4666767 | Dispensers for the controlled release of pest controlling agents and method for combatting pest therewith | 1987 |
| US6593299 | Compositions and methods for controlling pests | 2003 |
| US4296042 | Preparation of unsaturated aliphatic insect pheromones using cyclic phosphonium ylids | 1981 |
Physical Data
| Appearance | Colorless or light yellow oil |
| Flash Point | 102.3±20.4℃ |
| Boiling Point | 334.8±21.0℃ (760 Torr) |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Coupling Nuclei | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | 1H | chloroform-d1 | 300 |
| Chemical shifts | 13C | chloroform-d1 | 76 | |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 500 | |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 125 | |
| Chemical shifts | 1H | CDCl3 | 400 | |
| Chemical shifts | 13C | CDCl3 | ||
| Spectrum | CDCl3 | 400 | ||
| Chemical shifts | 1H | CCl4 | ||
| Spin-spin coupling constants | CCl4 | |||
| Spin-spin coupling constants | CDCl3 | |||
| NMR |
| (Z,E)-9,12-TETRADECADIENYLACETATE CAS#: 30507-70-1 NMR | 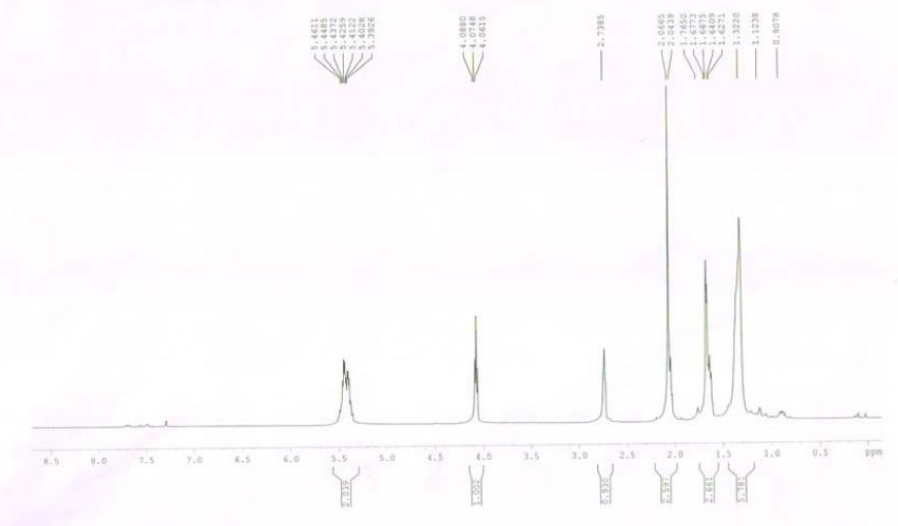 |
| (Z,E)-9,12-TETRADECADIENYLACETATE CAS#: 30507-70-1 CNMR | 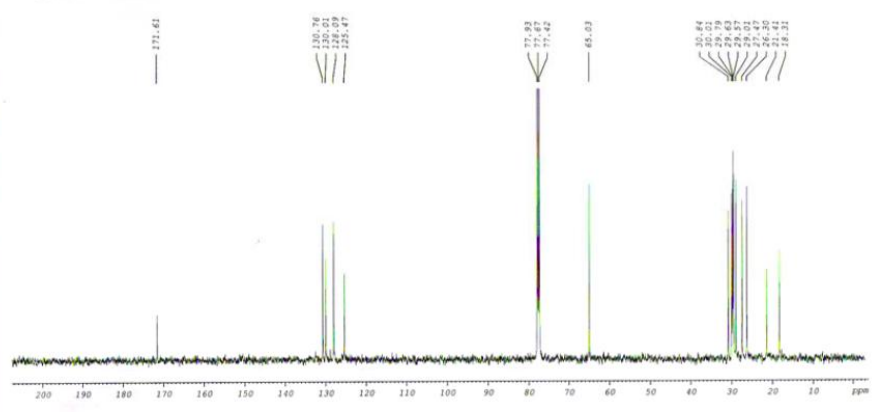 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands | gas | 3017 – 964 cm**(-1) |
| Bands | neat (no solvent) | 2960 – 730 cm**(-1) |
| Bands | CCl4 | 3010 – 720 cm**(-1) |
| Bands | neat (no solvent) | 2950 – 730 cm**(-1) |
| Bands | neat (no solvent) | 1745 cm**(-1) |
| Bands | neat (no solvent) | 3010 – 720 cm**(-1) |
| IR |
| Description (Mass Spectrometry) |
| gas chromatography mass spectrometry (GCMS), electron impact (EI), spectrum |
| high resolution mass spectrometry (HRMS), fast atom bombardment (FAB), spectrum |
| spectrum, chemical ionization (CI) |
| spectrum |
| Description (UV/VIS Spectroscopy) |
| UV/VIS |
Route of Synthesis (ROS)
| Conditions | Yield |
| With hydrogen; sodium tetrahydroborate; nickel diacetate; ethylenediamine In ethanol Ambient temperature; | 85% |
| With quinoline; hydrogen; Lindlar’s catalyst In hexane at -10℃; | 55.5% |
| With hydrogen; P-2Ni | |
| With quinoline; hydrogen; Lindlar’s catalyst In hexane at -10℃; Yield given; |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (50.56%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P264, P280, P302+P352, P321, P332+P313, and P362 (The corresponding statement to each P-code can be found at the GHS Classification page.) For more detailed information, please visit ECHA C&L website |
| Source: European Chemicals Agency (ECHA) License Note: Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: “Source: European Chemicals Agency, http://echa.europa.eu/”. Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. License URL: https://echa.europa.eu/web/guest/legal-notice Record Name: (1-Cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino-carbenium hexafluorophosphate URL: https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/213446 Description: The information provided here is aggregated from the “Notified classification and labelling” from ECHA’s C&L Inventory. Read more: https://echa.europa.eu/information-on-chemicals/cl-inventory-database |
Other Data
| Transportation | No data available |
| Under the room temperature and away from light | |
| HS Code | No data available |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 252.397 |
| logP | 6.543 |
| HBA | 2 |
| HBD | 0 |
| Matching Lipinski Rules | 3 |
| Veber rules component | |
| Polar Surface Area (PSA) | 26.3 |
| Rotatable Bond (RotB) | 12 |
| Matching Veber Rules | 1 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| pX | Parameter | Value (quant) | Unit |
| 5.12 | Ki (inhibition constant) | 7.58 | μM |
| percentage(Relative fluorescence) | 38.09 | % |
| 1 of 11 | Effect | pheromone |
| Assay Description | Effect : EAG response Target : antenna of Lacinipolia renigera, bristly cutworm Bioassay : title comp. isolated from volatile subst. emitted by adult female bolas spiders Mastophora hutchinsoni and collected during the period of mid-August through October, 1998, between 8 and 10 PM; gas chromatography (GC); electroantennography (EAG) proximal end of a male L. renigera antenna was placed into a pool of an insect saline solution; EAG response to title comp. was recorded and compared with simultaneously obtained GC data; effects of title comp. of synthetic and natural origins compared | |
| Results | GC-EAG peak of title comp. was identified using DB-Wax and DB-5 capillary columns | |
| 2 of 11 | Biological material | Ephestia kuehniella |
| Assay Description | Effect : insect attractant Example 7; Exosex2 SPL was formulated from 1% pheromone (Z9E12-14Ac) , 0.5% flow agent, 19.5% paraffin wax and 79% carnauba wax. This powder was compacted into Ig pellets.In a commercial flour mill in Andover, UK the following 2 treatments were compared during the control of theMediterranean | |
| Results | 45.91% mean trap catches at 0 DAT (26.33% in control); 26.55% mean trap catches at 60 DAT (26.0% in control); 4.27% mean trap catches at 136 DAT (54.0% in control) | |
| 3 of 11 | Biological material | Ephestia kuehniella |
| Assay Description | Effect : insect attractant Bioassay : Example 7; Exosex2 SPL was formulated from 1% pheromone (Z9E12-14Ac) , 0.5% flow agent, 19.5% paraffin wax and 79% carnauba wax. This powder was compacted into Ig pellets.In a commercial flour mill in Andover, UK the following 2 treatments were compared during the control of theMediterranean | |
| Results | 45.91% mean trap catches at 0 DAT (26.33% in control); 26.55% mean trap catches at 60 DAT (26.0% in control); 4.27% mean trap catches at 136 DAT (54.0% in control) potential area of application: agro | |
| 4 of 11 | Assay Description | Effect : electrophysiological Target : Spodoptera littoralis, Egyptian armyworm Bioassay : single sensillum recording (SRR); whole insect preparations; standard tip recording technique |
| Results | significant response; recording | |
| 5 of 11 | Effect | Behavioural Symptoms |
| Assay Description | Target : Spodoptera littoralis, Egyptian armyworm Bioassay : behavior: close approach to the lure (ca. 10 cm) laboratory colony; insects on first and second scotophase; glass wind tunnel 180 cm long, 55 cm wide, and 50 cm high; 58-W red fluorescence light; light intensity: 3 lux; airspeed 45-55 cm/sec; 22 deg C; 60 percent RH | |
| Results | response: ca. 5 to 55 percent; max. effect at 1000 μg | |
| 6 of 11 | Assay Description | Effect : electrophysiological Target : Spodoptera descoinsi Lalanne-Cassou & Silvain, noctuid moth Bioassay : responses of 10 males measured Lepidoptera: Noctuidae; electrophysiological activity of male antenna measured by electroantenographic technique (EAG); pure air as control; experiments were carried out on whole insect preparations |
| Results | corrected EAG response: 2.0 (control: ca. 0.8) | |
| 7 of 11 | Assay Description | Effect : electrophysiological Target : Spodoptera descoinsi Lalanne-Cassou & Silvain, noctuid moth Bioassay : responses of 10 males measured; LM: 11-40 hairs, MH: 2-3 hairs Lepidoptera: Noctuidae; electrophysiological activity of male antenna measured by single sensillum recordings in long lateral – LM (A and B cells) and short medial hairs (MH); air as control; experiments were carried out on whole insect preparations |
| Results | response profile; action potentials (mV)/s: LM: <10 (B cells), ca. 37 (A cells), MH: ca. 40 (control: <10) | |
| 8 of 11 | Assay Description | Effect : electrophysiological Target : Spodoptera latifascia (Walker), noctuid moth Bioassay : responses of 10 males measured Lepidoptera: Noctuidae; electrophysiological activity of male antenna measured by electroantenographic technique (EAG); pure air as control; experiments were carried out on whole insect preparations |
| Results | corrected EAG response: 2.5 (control: ca. 0.8) | |
| 9 of 11 | Assay Description | Effect : electrophysiological Target : Spodoptera latifascia (Walker), noctuid moth Bioassay : responses of 10 males measured; LM: 11-30 hairs, MH: 10-22 hairs Lepidoptera: Noctuidae; electrophysiological activity of male antenna measured by single sensillum recordings in long lateral – LM (A and B cells) and short medial hairs (MH); air as control; experiments were carried out on whole insect preparations |
| Results | response profile; action potentials (mV)/s: LM: ca. 37 (A cells), <10 (B cells), MH: 20 (control: <10) | |
| 10 of 11 | Results | pheromone of Cadra cautella (almond moth) and Plodia interpunctalla (Indian meal moth) |
| 11 of 11 | Results | pheromone component of various butterflys, inhibitor effects of various butterflys |
| Use Pattern |
| (Z,E)-9,12-TETRADECADIENYLACETATE CAS#: 30507-70-1 Controlling Parapediasia teterrella |
| (Z,E)-9,12-TETRADECADIENYLACETATE CAS#: 30507-70-1 Controlling Spodoptera exempta |
Related Chemicals
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Watson Bio Ltd | https://www.watson-bio.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |

![Structure of 4-[4-(acetyloxy)phenyl]-2-butanone CAS 3572-06-3](https://www.chemwhat.com/wp-content/uploads/2017/11/Structure-of-4-4-acetyloxyphenyl-2-butanone-CAS-3572-06-3-150x89.png)